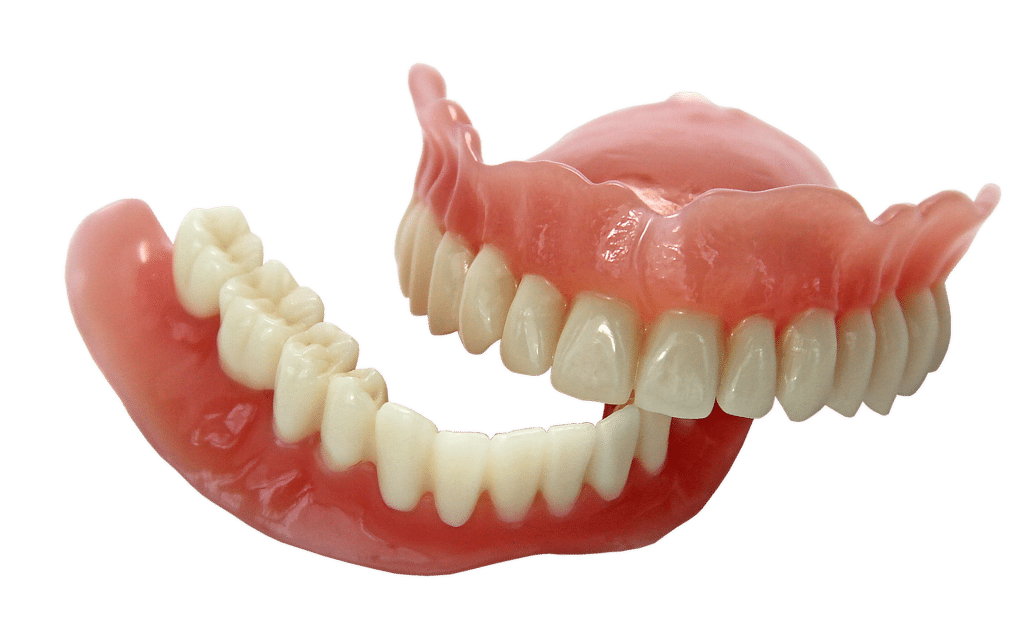
New FDA Product Code and Classification PZY for 3D Printed Denture Teeth
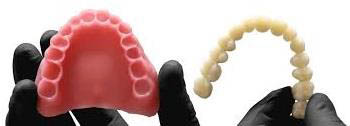
The dental laboratory industry continues to incorporate CAD/CAM automated manufacturing while the FDA is making clear their position on some regulatory requirements for these laboratory manufacturing processes.
The FDA’s Product Classifications are giving us light on 3D Printing manufacturing requirements.
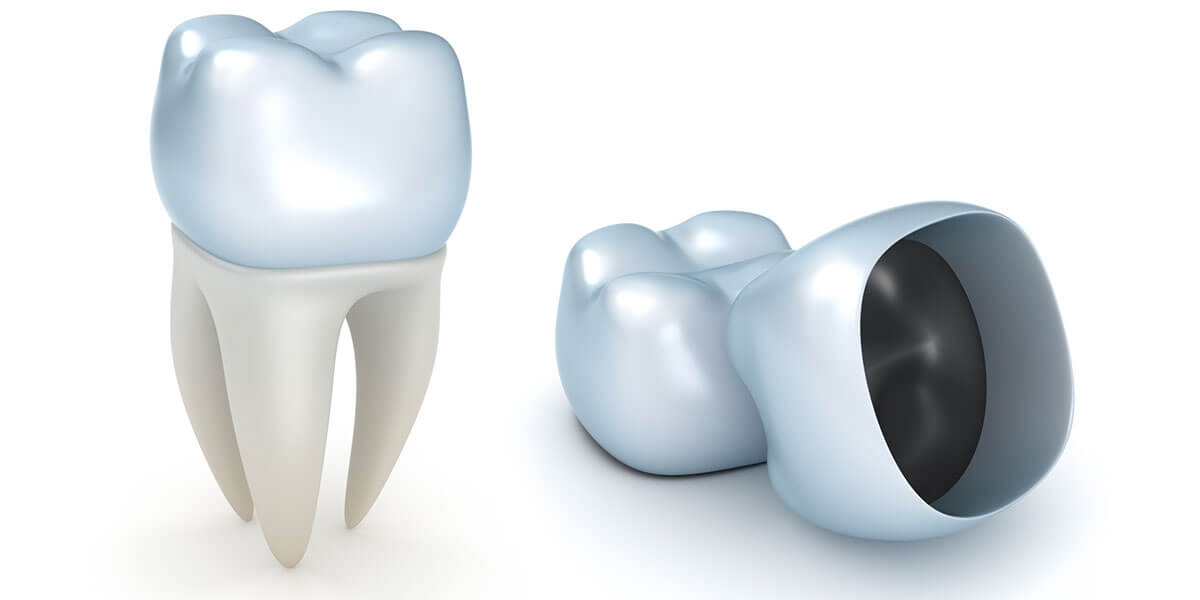
The FDA’s product classification for 3D printed denture teeth for the fabrication of preformed resin denture teeth for use in a denture base (Product Code PZY) is very straight forward.
- The product is a Class 2 device
- 510(k) exempt
- Requires to be produced under the Quality System (QS) Regulation
This all breaks down to good news for dental laboratories that 3D print dentures. The only requirements are that dental labs must have a Quality Management System (QMS) in place and use 510(k) cleared resin to print the denture teeth.
If you are looking for more information or need a QMS
call on us. 916-844-5267