3 Methods used by the FDA to Add and Modify CAD/CAM Restorations Requirements
Below are 3 different methods used by the FDA to deliver information of how they modified requirements and regulations. The FDA introduced the new Product Code PZY for the Additively Manufactured, Preformed Resin Denture Tooth. ...
The New FDA Regulatory Requirements Are Becoming Clear
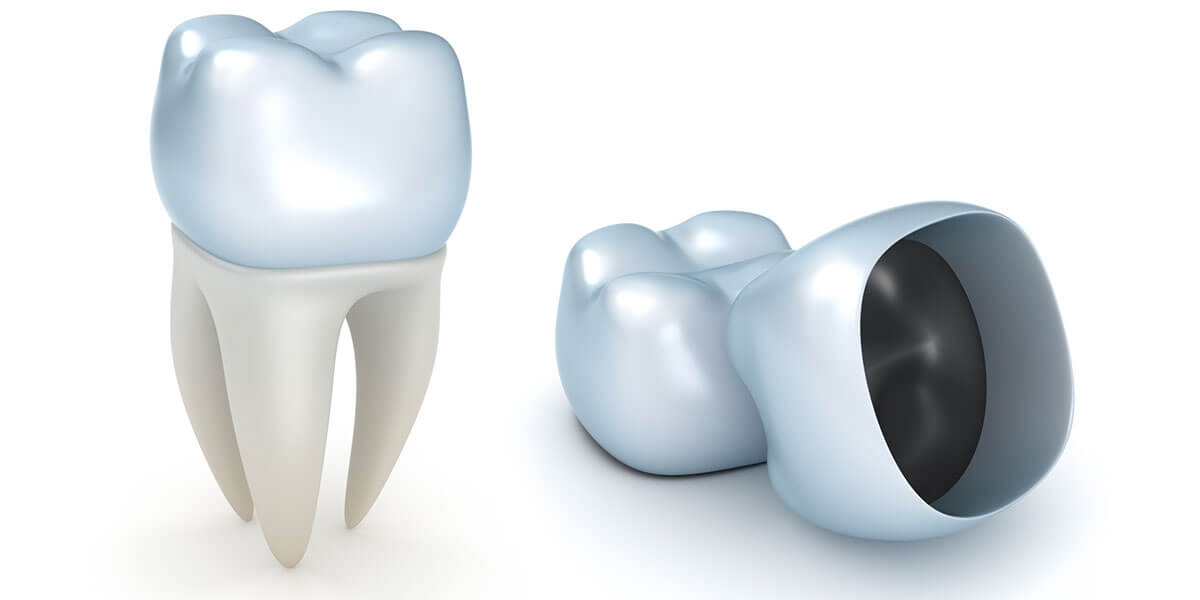
With the dental laboratory industry continuing to incorporate CAD/CAM automated manufacturing, the FDA is making changes to some regulatory requirements for CAD/CAM restorations and most likely more requirement changes to come. The FDA’s Product Classifications ...
The FDA Requirement For Orthodontic Sequential Aligners
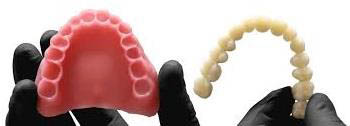
Many dental laboratories are receiving requests from dentists regarding the service of providing Orthodontic Clear Aligners...
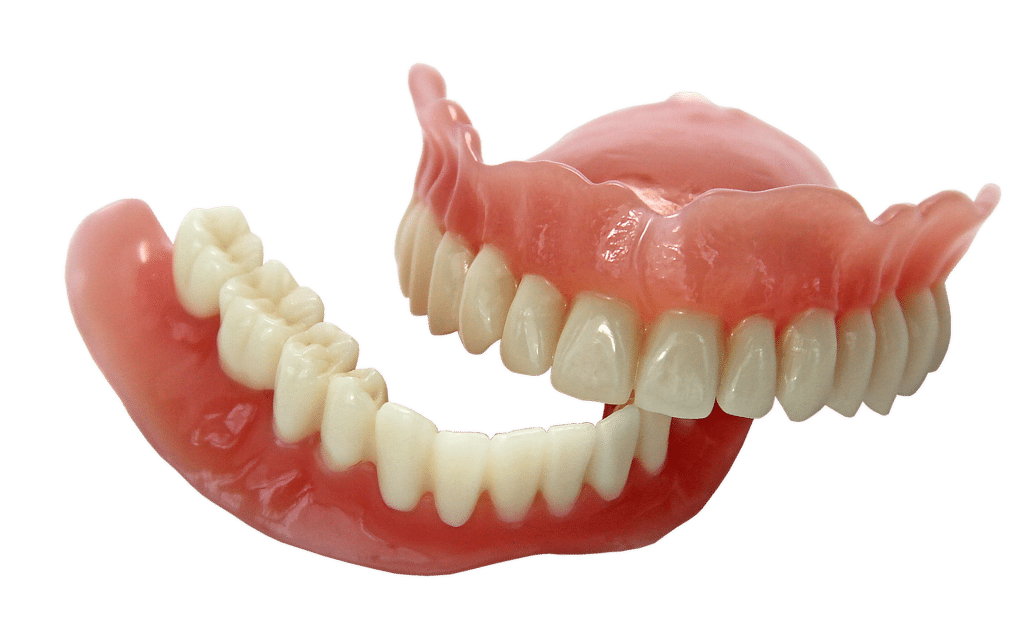
New FDA Product Code and Classification PZY for 3D Printed Denture Teeth
The dental laboratory industry continues to incorporate CAD/CAM automated manufacturing while the FDA is making clear their position on some...
Grow Your Laboratory with the Understanding of the FDA Requirements
Grow your laboratory with a clear strategy to comply with the FDA and incorporate all the services that are now available to offer your clients...